HOW DIAMONDS ARE FORMED
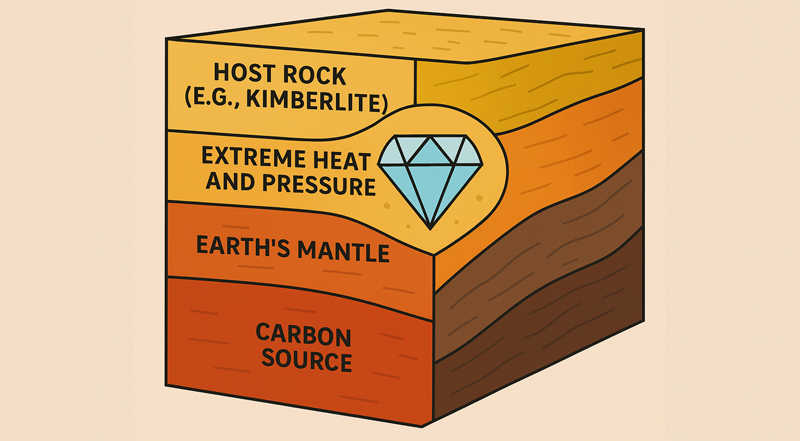
1. Carbon Source (Deep in the Mantle)
Diamonds begin as carbon atoms deep within the Earth’s mantle — around 140–200 kilometers below the surface.
The carbon may originate from:
Primitive carbon in the mantle
Subducted organic material (from ancient sea floors)
2. Earth’s Mantle
This is where diamonds actually crystallize.
Under conditions of extreme pressure (45–60 kilobars) and high temperatures (900–1,300°C), the carbon atoms bond in a cubic structure — forming diamond crystals. This process occurs over millions to billions of years.
3. Extreme Heat and Pressure Zone
This layer highlights the unique geological window where diamonds can form and remain stable.
If temperature is too high, the diamond will revert to graphite. Stability is very narrow, which is why natural diamonds are rare.
4. Host Rock (e.g., Kimberlite)
Diamonds are transported from the deep mantle to the surface by violent volcanic eruptions, forming kimberlite or lamproite pipes.
These pipes are carrot-shaped volcanic conduits that cool quickly, preserving the diamonds on their way up.
Key Pages: Create & Customize Jewelry
Key Pages: Diamond Jewley From the Atelier
Essential Diamond & Jewelry Education
Important Links: Support & Policies
MyAntwerpDiamonds.com /
Some email responses from us may be filtered as spam or blocked altogether. To ensure you receive our emails, please provide your telephone or WhatsApp number for verification.